
Optimizing lung cancer care for better patient outcomes
A case for faster diagnosis, biomarker testing, and treatment
The challenge
- Lung cancer is the most common cancer in Canada and the leading cause of cancer mortality for both sexes. Survival rates are among the lowest for all cancer types.1
- Lung cancer often gets diagnosed at an advanced stage. At this point, curative treatment options are limited, so a timely diagnosis has profound implications for a patient’s prognosis.
- Clinical practice guidelines consistently recommend prompt evaluation, diagnosis, and treatment of patients with suspected lung cancer, yet time intervals from referral to diagnosis or treatment vary significantly, both between and within hospitals.
- Lung cancer mortality is 5 to 10 per cent per month following diagnosis.2, 3 Delays in diagnostic testing translate to more patients not surviving long enough to receive treatment.
The hope of innovative therapies
- The past decade has seen an evolution of care in oncology treatment, from chemotherapy to targeted therapy and immuno-oncology.
- A multitude of potential targetable gene alterations have been identified in lung cancer. Timely initiation of appropriate, targeted treatment can improve survival rates and quality of life.
- Targeted therapies require timely biomarker testing. Patients with lung cancer experience a rapid deterioration in their health status, so their window of time for treatment eligibility is small. Expedited biomarker analysis allows them to receive potentially life-saving treatment in time.
- Today, pathology plays an ever-important role in the lung cancer patient-care trajectory. It is integral to multidisciplinary planning of diagnosis and treatment and to minimizing delays and enhancing patient outcomes.
Pathology at Institut universitaire de cardiologie et de pneumologie de Québec – Université Laval (IUCPQ-UL) lung cancer network: A model of care
- IUCPQ-UL has developed an optimized approach for diagnosis and molecular testing of lung cancer on a supra-regional basis to reduce the time from referral to treatment to 26 days (see Figure 1).
- This contrasts with the provincial median of 56 days (interquartile range from 34 to 81 days).4
Figure 1
IUCPQ-UL lung cancer patient trajectory
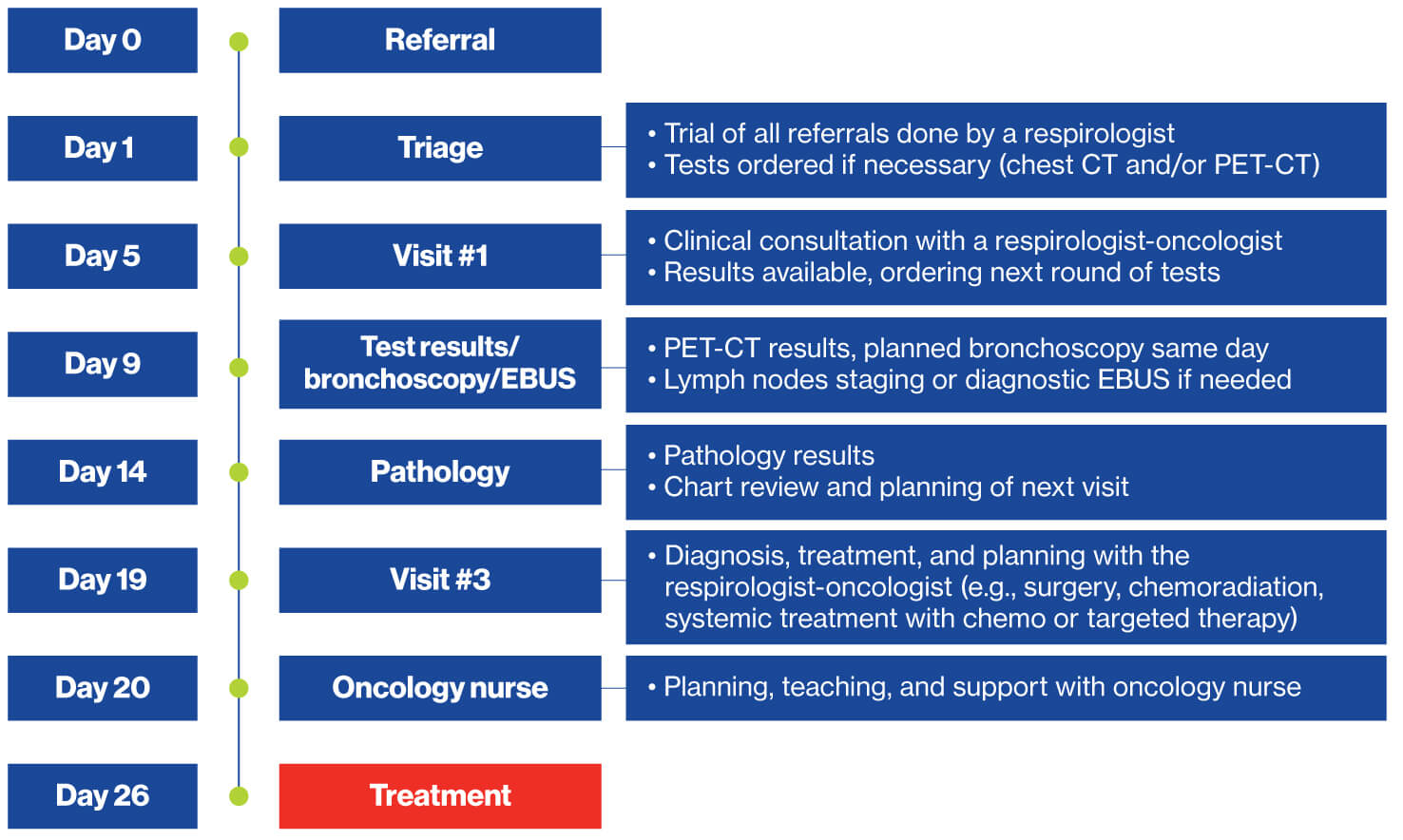
Sources: IUCPQ-UL; The Conference Board of Canada.
- Pathology plays a pivotal role in this pathway. An optimized workflow expedites processing and ensures low insufficiency rates for tissue samples.
- Despite high volumes, the pathology turnaround time is 2.6 days. This expedited processing is critical for patients in advanced stages of cancer, where molecular pathology is needed to determine the best course of treatment.
- IUCPQ-UL excels at all performance targets set by the Ministry of Health and Social Services (MSSS) (see Table 1).
Table 1
Turnaround times in pathology at IUCPQ-UL in 2019 relative to MSSS targets
| Test | MSSS target 1 | IUCPQ-UL performance | MSSS target 2 | IUCPQ-UL performance |
|---|---|---|---|---|
| Cytology | 5 days | 2.1 days | 80% | 99% |
| Biopsy | 7 days | 2.1 days | 80% | 100% |
| Surgery | 12 days | 2.4 days | 80% | 100% |
| Biomarkers | 10 days | 2.2 days | 100% | 100% |
Sources: IUCPQ-UL; The Conference Board of Canada.
- Tissue adequacy for molecular pathology is high, with extremely low rates of insufficient samples (1.5 per cent compared with rates as high as 10 per cent in other centres).5
- Compared with averages obtained from about 30 non-specialized regional hospitals and centres, IUCPQ-UL showed:6
- 71 per cent lower rate of sample insufficiency
- total cost savings of about $57 per biopsy (36 per cent lower)
- technical cost savings of about $50/biopsy (45 per cent lower)
- cost savings associated with pathologist honoraria of about $12/biopsy (21 per cent lower)
Success factors of IUCPQ-UL
- high level of specialized expertise localized in a small hospital
- close interactions within an interdisciplinary team, including respirologist-oncologists, pathologists, surgeons, radiologists, and nurses
- standardized protocols and homogenous practice (e.g., clear sequence of steps, check-in/verification points, and quality-control mechanisms for optimal pathology processing)
- reserved time slots for imaging exams, bronchoscopies, and biopsies
- dedicated nurses to document follow-up investigations
Limitations
The impact of the optimized IUCPQ-UL pathology laboratory on survival rates, access to precision therapies, and patient-reported outcomes is currently unknown. It took years for the Centre to establish this level of efficiency; hence, long-term outcome data are not yet available.
Conclusion
The IUCPQ-UL standardized approach to lung cancer care can serve as a blueprint for a health care infrastructure that supports molecular testing and optimized referral-to-treatment timeframes. The model is consistent with international best practices and recommendations for lung cancer care.
This approach could potentially be adopted by other institutions around the world. It would support timely molecular testing and rapid turnaround, producing results necessary to avoid delays in starting first-line treatment.
Acknowledgments
This case example was completed by request of the Quebec Cancer Coalition to highlight best practices in oncology diagnostics and their potential impact on patient outcomes.
1 Canadian Cancer Society, “Lung Cancer Statistics,” accessed August 6, 2020.
2 Roy S. Herbst and others, “Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial,” Lancet 387, no. 10027 (2016): 1540–1550, accessed October 29, 2020.
3 Alice T. Shaw and others, “Crizotinib versus chemotherapy in advanced ALK-positive lung cancer”, New England Journal of Medicine 368, no. 25 (2013): 2385–2394, accessed October 29, 2020 (published correction appears in New England Journal of Medicine 373, no. 16 (2015): 1582). doi:10.1056/NEJMoa1214886.
4 C. Labbé and others, “Wait times for diagnosis and treatment of lung cancer across the province of Quebec, Canada,” Journal of Thoracic Oncology 13, no. 10 (2020), S615–S616, accessed October 29, 2020.
5 Personal communication with IUCPQ-UL.
6 All tissue quality and costs data provided by IUCPQ-UL and based on 2,511 pathology reports from in-house and sent-in samples analyzed for lung cancer biomarkers at IUCPQ-UL.


Comments